Antibacterial activity of Cinnamomum zeylanicum aqueous extract against Proteus mirabilis isolated from Urinary Tract Infections in Kerbala province
DOI:
https://doi.org/10.59675/P315Keywords:
Proteus mirabilis, urinary tract infections, virulence factors, Cinnamomum zeylanicumAbstract
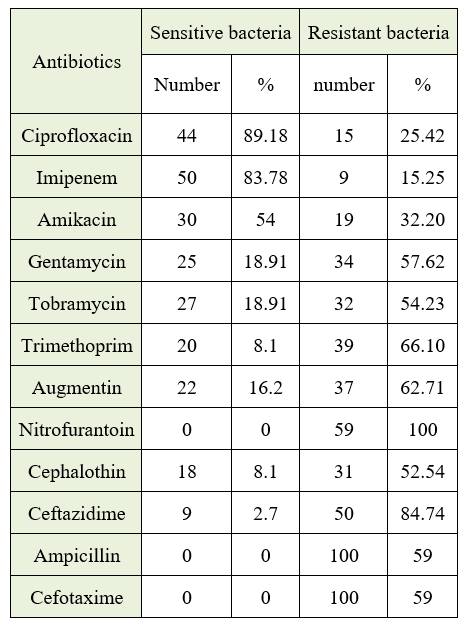
Proteus mirabilis from urinary tract infections (UTIs) is to be isolated as well as detected in the presented work. Proteus mirabilis resistance to specific antibiotics is being studied, and the antibacterial activity regarding a cold aqueous extract of Cinnamomum zeylanicum against Proteus mirabilis isolated from UTIs is being tested. 150 patients with diuresis for UTIs comprise the study sample. Thus, 12 (8%) of Proteus mirabilis were isolated. The majority of bacterial isolates tested with such antibiotics exhibited resistance to them. The research's findings indicate that the aqueous extract of Cinnamomum zeylanicum contains active components such as tannins, glycosides, saponins, resins, and phenols in varying amounts, as well as the absence of flavones and phenols. Thus, the bacterial isolates have the greatest impact on concentration (75 mg/ml). Therefore, compared with the control factor, which contains sterile distilled water, the concentration (75 mg/ml) had the highest and lowest diameters of 30 mm and 22 mm, respectively; the concentration (50 mg/ml) had the highest and lowest diameters of 28 mm and 18 mm, respectively; the concentration (25 mg/ml) had the highest diameter of 26 mm and the least diameter of inhibition was 16 mm; and lastly, about the concentration (12.5 mg/ml), the highest and lowest diameters were 23 mm and 12 mm, respectively.
The results of the investigation of some virulence factors showed that 59(100%) isolates can form a swarm, 48(81.35%) isolates can produce biofilm, 50(91.52%) isolates can produce hemolysin, and 24(100%) isolates can produce urease enzymes. Proteus mirabilis is one of the pathogens of UTIs.
References
Verrier JK. Screening after urinary tract infection in childhood. Arch Dis Child. 2000; 2:123-124.
Stamm EW, Hooton TM, Johnson JR, et al. U.T.I. From Pathogenesis to Treatment. J Infect Dis. 1989;15g:400-405. DOI: https://doi.org/10.1093/infdis/159.3.400
England AC, England TK. Pediatrics, Urinary tract Infection and Pyelonephritis. Emed.Com, Inc. J Med. 2002;2[6].
Tangho EA, McAninch JW. Bacterial infection of the genitourinary tract in General Urology. In: Smith E, editor. United States of America: McGraw-Hill Companies Inc.; 2004. p. 203-227.
Masson P, Matheson S, Webster AC, Craig JC. Meta-analyses in prevention and treatment of urinary tract infections. Infect Dis Clin North Am. 2009;23[2]:355-385. DOI: https://doi.org/10.1016/j.idc.2009.01.001
Sobel JD, Kaye D. Urinary tract infections. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 5th ed. Philadelphia: Churchill Livingstone; 2000. p. 773-805.
Mims C, Playfair J, Roitt I, Wakelin D, Williams R, Anderson RM. Medical Microbiology. London: Mosby; 1993.
Smith PW, Siep CW, Schaefer SC, Dixon CB. Microbiologic survey of long-term care facilities. Infect Control Epidemiol. 2000;28:8-13. DOI: https://doi.org/10.1016/S0196-6553(00)90005-1
Nicolle LE. The chronic indwelling catheter and urinary tract infection. Long-Term Care Facility Residents in Infect Control Hosp. 2001;22:316-322. DOI: https://doi.org/10.1086/501908
Fünfstück R, Ott U, Naber KG. International Journal of Antimicrobial Agents. 2006;28(Supplement 1):72-77. DOI: https://doi.org/10.1016/j.ijantimicag.2006.05.004
Meyrier A. Urinary tract infection. The Textbook of Medical Microbiology. 3rd ed. Stough, London; 2000. p. 124-150.
Abrutyn F, Brein J, Mossey J. Does treatment of asymptomatic bacteriuria in older ambulatory women reduce subsequent symptoms of urinary tract infections? J Am Geriatr Soc. 2000;44:293-295.
Li X, Zhao H, Lockatell V, Drachenberg CB, Johnson DE, Mobley HLT. Visualization of Proteus mirabilis within the matrix of urease-induced bladder stones during experimental urinary tract infection. Infect Immun. 2002;70:389-394. DOI: https://doi.org/10.1128/IAI.70.1.389-394.2002
Li X, Mobley HLT. Vaccines for Proteus mirabilis in urinary tract infection. Int J Antimicrob Agents. 2002;6(190):461-465. DOI: https://doi.org/10.1016/S0924-8579(02)00102-4
McCann J. Herbal Medicine Handbook. 2nd ed. Philadelphia: Lippincott; 2003.
Baron EJ, Finegold SM. Microorganisms encountered in urinary tract infections. In: Bailey & Scott's Diagnostic Microbiology. 9th ed. Mosby Co; 1994.
Penn N. Antibacterial activity examined for urinary tract infection. Med Lab Sci. 1987;44:41-44.
National Committee for Clinical Laboratory Standards (NCCLS). Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement. M100-S17. USA: NCCLS; 2007.
Collee JG, Fraser AG, Marmian BP, Simmons A. Mackie and McCartney Practical Medical Microbiology. 14th ed. Churchill Livingstone Inc; 1996.
Forbes BA, Sahm DF, Weissfeld AS. Bailey and Scott's Diagnostic Microbiology. 11th ed. Mosby Inc.; 2002.
Liaw SJ, Lai HC, Ho SW, Wang WB. Inhibition of virulence factor expression and swarming differentiation in Proteus mirabilis by p-nitrophenylglycerol. J Med Microbiol. 2000;49:725-731. DOI: https://doi.org/10.1099/0022-1317-49-8-725
Desmukh SD, Borle MN. Studies on the insecticidal properties of indigenous plant products. India J Enth Pharm. 1975;37[1]:11-18.
Connors KA. Pharmaceutical Analysis. New York: 1982. p. 499-506.
Perez C, Pauli M, Bazergue P. An antibiotic assay by the agar-well diffusion method. Acta Biologiae et Medicinae Experimentalis. 1990;15:113-115.
Ali FA. Resistance of bacteria causing urinary tract infection to antibiotics in Tikrit city. MSc Thesis, Education College for Women, University of Tikrit, Iraq; 1998.
Al-Jouburi ROS. Screening of Beta-lactamase Enzymes in Some Gram-negative and Positive Bacteria Clinically Isolated and the Effect of Some Prepared Chemical Compounds on Them. MSc Thesis, College of Science, University of Mosul, Iraq; 2000.
Chevins C. General Description of Urinary Tract Infection. Nidus Information Services, Inc. 41 East 11th Street, 11th Floor; 2001.
Sligh JD, Timbury MC. Notes on Medical Bacteriology. Churchill Livingstone Inc; 1994.
Abrutyn F, Brein J, Mossey J. Does treatment of asymptomatic bacteriuria in older ambulatory women reduce subsequent symptoms of urinary tract infections? J Am Geriatr Soc. 2000;44:293-295. DOI: https://doi.org/10.1111/j.1532-5415.1996.tb00917.x
Shapiro T, Dalton M. The prevalence of urinary tract infection and sexually transmitted disease in women with symptoms of a simple urinary tract infection stratified by low colony count criteria. Acad Emerg Med. 2005;12[1]:38-99. DOI: https://doi.org/10.1197/j.aem.2004.08.051
Adams RD, Petersdorf RG, Braunwald DE, Isseibacher KJ, Martin SB, Wilson JD. Diseases of the Kidney & Urinary Tract in Harrison's Principles of Internal Medicine. 10th ed. McGraw-Hill International Company; 1984.
Al-Charrakh AH. Bacteriological and Genetic Study on Extended-Spectrum β-Lactamases and Bacteriocins of Klebsiella Isolated from Hilla City. PhD Thesis, College of Science, Baghdad University; 2005.
Al-Autbi DAK. Bacteriological Study of Some Species of Enterobacteriaceae Isolated from Hospital Birth Rooms in Baquba City. Thesis, Diyala University; 2013.
Khalaf SH, Kadhum ATB. Isolation and Pathogenic Study on Proteus mirabilis. Baghdad Journal of Science. 2009;1[7]:317-326. DOI: https://doi.org/10.21123/bsj.7.1.317-426
Abdulghani ST. The Outcome of the Misuse of 3rd Generation Cephalosporins in Fallujah City, West of Iraq. Al-Anbar Med J. 2012;10[2]:46-50. DOI: https://doi.org/10.33091/AMJ.0601022012
Kezeer EG. Bacteriological Study of Otitis Externa and Susceptibility to Antimicrobial Agents. J Fac Med Baghdad. 2007;49[2]:281-283.
Al-Baytti SAK. A Bacteriological and Genetic Study of Proteus spp. Causing Urinary Tract Infection in Tikrit District. Thesis, University of Tikrit; 2010.
Jaloob AA, Gafil FA. Effect of Some Antibiotics on Aerobic Pathogenic Bacteria Causing Otitis Media and Urinary Tract Infection in Al-Manathera City in Iraq: A Comparative In Vitro Study. Q M J. 2012;8[13]:156-168. DOI: https://doi.org/10.28922/qmj.2012.8.13.156-168
Winokur PL, Canton R, Casellas JM, Legakis N. Variations in the performance of strains expressing an extended-spectrum β-Lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin Infect Dis. 2001;32[2]:94-103. DOI: https://doi.org/10.1086/320182
Ling JM, Lam AW, Chan EW, Cheng AF. What have we learned from community-acquired infections in Hong Kong? J Antimicrob Chemother. 2003;51[4]:895-904. DOI: https://doi.org/10.1093/jac/dkg167
Lazarevic G, Petreska D, Povlovic S. Antibiotic sensitivity of bacteria isolated from the urine of children with UTI from 1986-1995. Srp Arh Celok Lek. 1998;126:423-429.
Al-Taai HRR. Bacteriological, Biochemical, and Molecular Study of Proteus mirabilis Isolated from Urinary Tract Infections in Some Hospitals of Baghdad City. Thesis, Al-Mustansiriya University; 2005.
Al-Bassam WW, Al-Kazaz AK. The Isolation and Characterization of Proteus mirabilis from Different Clinical Samples. J Biotechnol Res Center. 2013;7[2]:24-30. DOI: https://doi.org/10.24126/jobrc.2013.7.2.261
Levinson W, Jawetz E. Enterobacteriaceae. In: Medical Microbiology and Immunology. 6th ed. McGraw-Hill Company, USA; 2000. p. 122.
Barun PB, Bitter W, Tommassen J. Activation of Pseudomonas aeruginosa elastase by triggering dissociation of the propeptide-enzyme complex. Microbiol. 2000;146:2565-2572. DOI: https://doi.org/10.1099/00221287-146-10-2565
Burall LS, Harro JM, Li X, Lockatell CV, Himpsl SD, Hebel JR, Johnson DE, Mobley HLT. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect Immun. 2004;72[5]:2922-2938. DOI: https://doi.org/10.1128/IAI.72.5.2922-2938.2004
Al-Ouqaili MTS, Al-Kubaisy SHM. Crystalline Biofilm Produced by Proteus mirabilis: An Overview on Their Formation Assays and Antimicrobial Interaction. Al-Anbar J Med. 2008;6[1]:33-42.
Perez C, Pauli M, Bazergue P. An antibiotic assay by the agar-well diffusion method. Acta Biologiae et Medicinae Experimentalis. 1990;15:113-115.
Almjalawi BSA, Al-Awade HAR, Al-Mafragy HS, Al Masaoodi NN. Antibacterial Activity of Capsicum annum L. Juice Against Klebsiella pneumonia Isolated from Respiratory Tract Infections. Iranian J War Public Health. 2022;14[2]:139-146.
Pfaller MA, Mujeeb I, Hollis RJ, Jones RN, Doern GV. Evaluation of the discriminatory powers of the dienes test and vibotyping as typing methods for Proteus mirabilis. J Clin Microbiol. 2000;38:1077-1080. DOI: https://doi.org/10.1128/JCM.38.3.1077-1080.2000
Al-Duliami AA, Nauman NG, Hasan AS, Al-Azawi ZH. Virulence Factors of Proteus mirabilis Isolated from Patients with Otitis Media in Baquba and its Peripheries. Diyala J Med. 2011;1[1]:69-75.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Academic International Journal of Pure Science

This work is licensed under a Creative Commons Attribution 4.0 International License.